How to Determine Limiting Reactant
Determine the number of moles of each reactant. A If the calculated MOLES NEEDED is greater than the MOLES HAVE for a given reactant then that reactant is the limiting reagent.

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube
Once you find the moles only convert one of them to the moles of the other reactant.

. Choose the reactant that produces the least amount of product. Divide by the coefficients of the hydrogen reactant 000134mole3 000045 mole. Calculate the number of moles of the required product from molar amounts of all reactants.
Use the amount of limiting reactant for calculating the amount of product produced. Limiting reactant is also what prevents a reaction from continuing because there is none left. Moles of HCl 025.
Finding the limiting reagent by calculating and comparing the amount of. The limiting reactant is the reagent compound or element to be totally consumed in a chemical reaction. So the limiting reagent in this reaction is the hydrogen because it gave the least number of moles.
An example is shown below-. So in the above problem o 2 is the limiting reactant because limiting reactant reactant that produces least ml of product. Parrott teaches you how to determine the limiting reactant in a chemical reaction.
Now use the moles of the limiting reactant to calculate the mass of the product. To determine the limiting reactant following steps should be taken. Remember to use the molar ratio between the limiting reactant and the product.
This is known as a limiting reactant. The other reactants are partially consumed where the remaining amount is considered in excess. Yes the percentage of yield can be calculated from the concept of limiting reactant.
Now use the moles of the limiting reactant. This free limiting reactant calculator assists you to calculate limiting reactant that goes for finishing during the reaction and makes a limited amount of product. In the light of chemistry.
183 g of silver chloride is collected as precipitate. Calculate the number of moles of the required product from molar amounts of all reactants. Calculate the number of moles H 2 3022400.
The limiting reactant may also be referred to as limiting reagent or limiting agent. Learn how to identify the limiting reactant in a chemical reaction and use this information to calculate the theoretical and percent yields for the reaction. The reactant used up first is known as the limiting reactant.
Theoretical yield is based on the calculation using the amount of limiting reactant 150 mol H 2. Calculate the number of moles H 2 given volume molar volume. To determine how much product Fe 3 O 4 will be made multiply the limiting reactant times the mole ratio of product to the limiting reactant and then multiply by the molar mass of the product.
Lastly if necessary calculate how much of the non-limiting agent is left in excess. The reactant that is not used up is referred to as the. In a given stoichiometry problem you will use this reactant to determine amount of product formed.
Follow us on Twitter Viking_Science. One reactant will be completely used up before the others. If 25 ml of 0320 M barium chloride takes part in a reaction with excess amount of silver nitrate and form the silver chloride precipitate.
The reactant that forms the least amount of product will be the limiting reactant. For example if you had a equation of 2h22o2----2h202 find the moles of h2 through o2 by multiplying. Postby anishathomas Thu Oct 11 2018 609 pm.
Lets go through the kinematics of the limiting reactions in the context below. Suppose you have the following chemical equation and you are asked to find the limiting reactant if the amount of sodium is 25g and that of chlorine is 40g. Used to determine limitingexcess reagent.
We will work out a couple examples together and at the end of the. Now use the moles of the limiting reactant to calculate the mass of the product. B If the calculated MOLES NEEDED is less.
Then that reactant is the limiting reagent. To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed. The way I calculate the limiting reactant is by first finding the amount of moles are in the reactants given.
This example problem demonstrates a method to determine the limiting reactant of a chemical reaction. In this video we will learn how to determine the limiting reactant of a chemical reaction. Those are called the excess reactants.
Divide the actual number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation. Get the number of moles of reacting substances from the given amounts of reactants.

How To Identify The Limiting Reactant In A Drawing Of A Mixture Chemistry Study Com

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
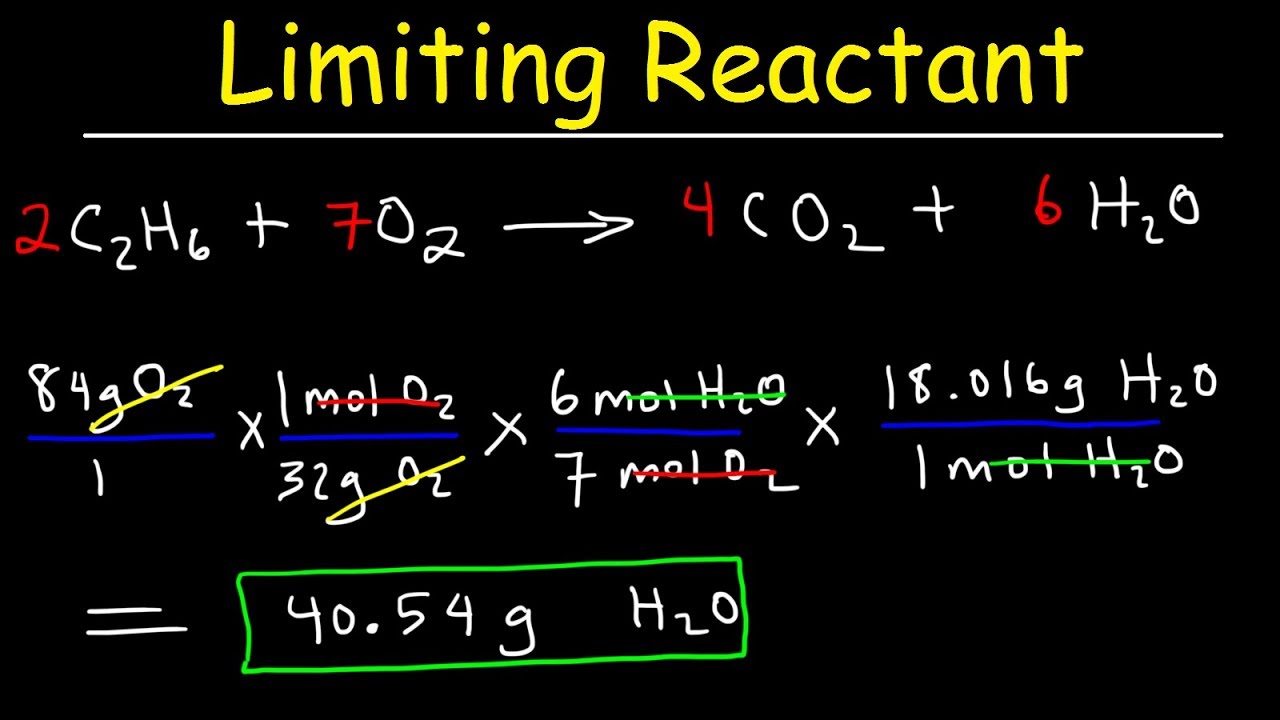
Limiting Reactant Practice Problems Youtube

Limiting Reagent Reactant Definition Examples And Problems

Limiting And Excess Reactant Stoichiometry Problems Youtube
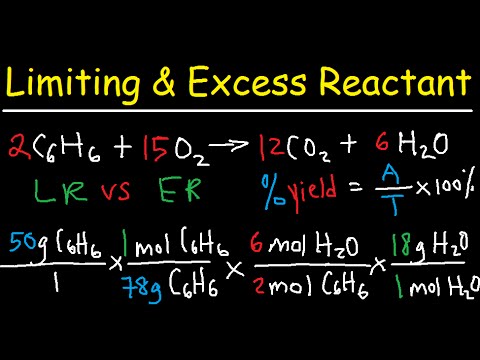
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube
Comments
Post a Comment